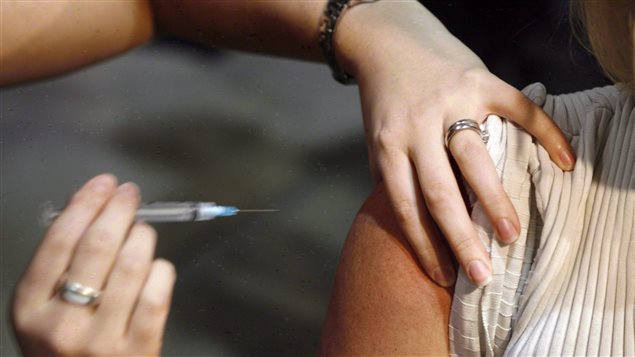
A company that makes much of Canada’s annual flu vaccine has received a warning from the U.S. Food and Drug Administration.
GSK Biologicals received a letter of warning from the FDA about conditions at its manufacturing facility at Ste-Foy in the province of Quebec where the company makes flu vaccine for the Canadian and U.S. markets.
The letter raised concerns about the purified water system at the plant: “Controls for the purified water system at your facility are inadequate to prevent bioburden and endotoxin excursions.”
It also raised other issues such as “You failed to assure that appropriate written procedures designed to prevent microbiological contamination of drug products purporting to be sterile, are established and followed.”
The FDA gave the company 15 days in which to address the problems.
“You should take prompt action to correct these deviations. Failure to promptly correct these deviations may result in regulatory action without further notice. Such actions may include license suspension and/or revocation.”
According to the Canadian Press news agency a statement from GSK says the company is making progress addressing the FDA’s concerns and is committed to working with the regulatory agency to fully resolve all outstanding issues.
More information:
FDA letter to GSK Biologicals – here
GSK website – www.gsk.ca
Canadian Press/Helen Branswel – Warning letter says GSK facility that makes Canada’s annual flu vaccine doesn’t meet current good manufacturing practices – here






For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.