A Quebec City-based biopharmaceutical company has begun Canada’s first clinical trials of its plant-derived COVID-19 vaccine candidate.
Officials with Medicago announced Tuesday that the first doses of the vaccine candidate were administered to 180 healthy volunteers – men and women aged 18 to 55 – on Monday as part of the Phase 1 clinical trials.
The clinical trial is a randomized, partially blinded study and will evaluate dosages of 3.75, 7.5 or 15 micrograms of the recombinant Coronavirus Virus-Like Particle (CoVLP) vaccine candidate alone or with an adjuvant in a prime-boost regimen, said in a statement Nathalie Landry, executive vice president of scientific and medical affairs at Medicago.
These virus-like particles prompt an immune response similar to that elicited by the natural virus, but they are non-infectious because they do not contain the genetic material the virus needs to replicate inside cells.
Instead of using a partially disabled live germ or the killed version of the germ that causes a disease, recombinant vaccines use specific pieces of the germ – like its protein, sugar or capsid – to elicit an immune response.
Recombinant vaccines, however, often require the use of an adjuvant, an ingredient used in some vaccines that helps create a stronger immune response in people receiving the vaccine.
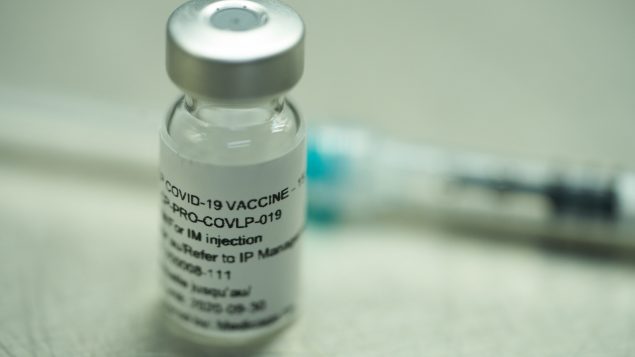
Vial of a vaccine candidate. Medicago expects to be able to manufacture approximately 100 million doses by the end of 2021. (CNW Group/Medicago)
The company hopes to begin the second phase of the clinical trials in October.
“We are thrilled to see our COVID-19 vaccine candidate enter the Phase 1 trial, and we look forward to obtaining safety and immunogenicity results in October,” said Landry.
“Our progress continues to demonstrate the value of Medicago’s unique plant-based vaccine technology.”
Medicago will be testing its vaccine candidate with two adjuvants separately – GSK’s proprietary pandemic adjuvant technology and Dynavax’s CpG 1018, Landry said.
An adjuvant can be of particular importance in a pandemic situation as it may boost the immune response and reduce the amount of antigen required per dose, allowing more vaccine doses to be produced and therefore contributing to protect the greatest number of people, said officials at Medicago.
“Creating a sufficient supply of COVID-19 vaccines within the next year is a challenge which will require multiple approaches, with different technologies,” said in a statement Dr. Bruce Clark, president and CEO of Medicago.
Medicago expects to be able to manufacture approximately 100 million doses by the end of 2021.
By the end of 2023, the construction of Medicago’s large-scale facility in Quebec City, will be completed. It is anticipated that this commercial facility will have the capacity to produce up to 1 billion doses of the COVID-19 vaccine annually, Clark said.






For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.