The Canadian government agency, Health Canada, has approved a new rapid test technology for COVID-19.
Being able to quickly detect a positive infection in someone quickly can save lives by more quick reaction in isolation and treatment to avoid the chance of inadvertent spreading of the virus. This could be very important especially in such places as long-term care facilities with their potentially vulnerable residents. The quick testing compares with current lab based detection where results can take days, or from remote communities a week, during which time an infected person not showing symptoms can potentially spread the virus.
The new technology developed by Spartan Bioscience of Ottawa is a small, hand-sized easily portable device that can determine if someone tests positive or negative for the virus, which is more technically known as SARS-CoV-2.
Positive tests can be determined in about 30 minutes, while determination of a negative test takes up to an hour.
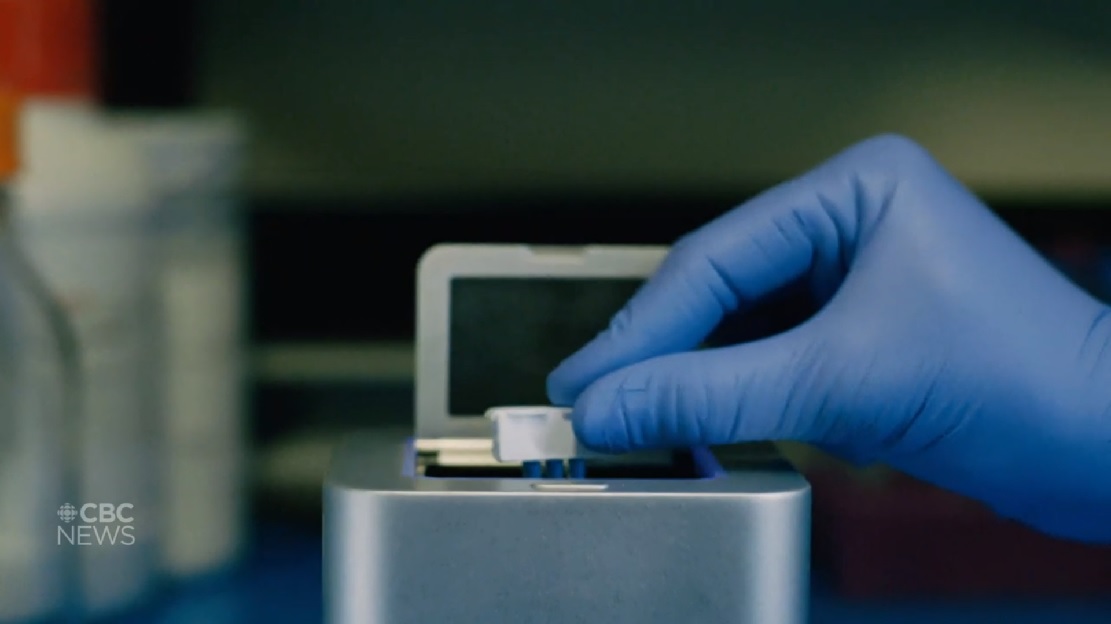
Developed from Spartan’s DNA test technology, the test kit for COVID-19 is inserted into the cube, the lid is closed and within an hour it can determine an individual’s positive or negative result for the virus (CBC)
Typically detection for COVID-19 requires the specialized equipment of a large laboratory, but the so-called ‘Spartan Cube’, is self contained. The technology was adapted from Spartan’s portable DNA testing technology to specifically test for COVID-19 genetic sequencing.
The test involves taking swabs from the nose or throat and can be performed by non-medical personnel such that it could be employed in places like airports or border crossings.
It is expected to be especially useful in communities outside large urban centres where previously samples had to be sent in to the larger centres which had the necessary testing equipment. The more distant or remote the community, the greater the advantage of this new technology.

Another advantage is that most of the production for the Spartan technology is done in Canada, such as L-D Tool & Die in Stittsville, Ont., will produce swabs, tubes and packaging for Spartan (Laurie Dickson via CBC)
Even in major centres the device can provide fast results without involving the laboratory which can continue with other important tasks.
The firm is currently ramping up production, but how quickly that can be achieved to meet demand is not yet known.
The province of Alberta has ordered 250 cubes and 100,000 test kits, Ontario has ordered 900,000 kits, Quebec 200,000 kits. The limitation of the device is that it can only perform one test at a time,
Another Canadian technology seeks rapid approval
A Windsor Ontario based company has also developed a COVID-19 test.
Even as Spartan has its Health Canada approval, Audacia Bioscience has applied this week for Health Canada approval for its COVID-19 test technology.
Claimed to give results within ten minutes, the test uses a couple of blood drops from a pin-prick dropped into a solution. Simply mixing the two in a small container gives can provide the results. The technology involves a cassette-like container only a little larger than a man’s thumb.
Rather than test for live virus, the technology determines the presence of antibodies to the virus in the blood. and is said to determine not only live virus infection, but also whether someone is assymptomatic or has recovered from the virus as antibodies remain in the bloodstream.
Both developers say their tests allow for easier, quicker, and inexpensive mass testing, something they say is needed to determine the extent of the infection to better plan on how to deal with it.
Several other firms around the world are also developing rapid test technologies for COVID-19
additional information-sources
CBC: A. Pfeffer: Apr 13/20: ‘Everyone wants them’-Rapid COVID-19 test kits made in Canada approved







For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.