There may be some good news for disgruntled Canadians exasperated with the country’s lagging COVID-19 vaccine rollout.
Canadian Press is reporting today that Canada could get more than one million additional vaccine doses by the end of March.
CP’s Mia Rabson quotes Procurement Minister Anita Anand telling the news agency that up to 1.1 million doses of AstraZeneca’s vaccine could arrive by the end of March and up to 3.2 million total by the end of June.
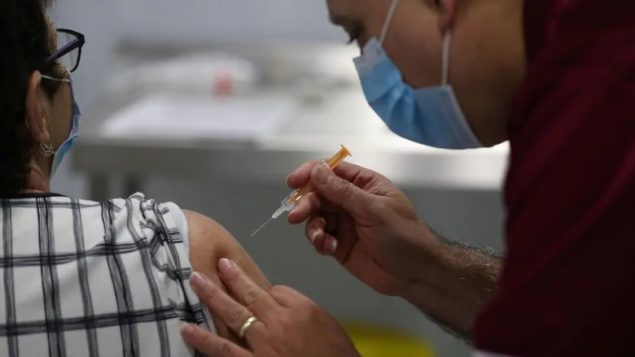
A person receives a dose of the Oxford-AstraZeneca COVID-19 vaccine at the Winter Gardens in Blackpool, U.K. (REUTERS/Peter Byrne)
They would come through a global vaccine sharing initiative known as COVAX that pools funds from wealthier countries to buy vaccines for themselves and for 92 low-and middle-income nations that can’t afford to buy on their own.
Canada, Rabson reports, contributed $440 million to COVAX in September, half of which secured doses for Canada directly from about nine vaccines that are participating in the program.
The other half goes into a pooled fund to buy doses for 20 per cent of the people in those 92 countries.
Rabson notes that the plan is subject to the kind of delays and shipments that Canada has been facing since vaccines began rolling out in December. (Unlike the Pfizer-BioNTech and the Moderna vaccines, AstraZeneca’s vaccine has yet to be approved by Health Canada.)
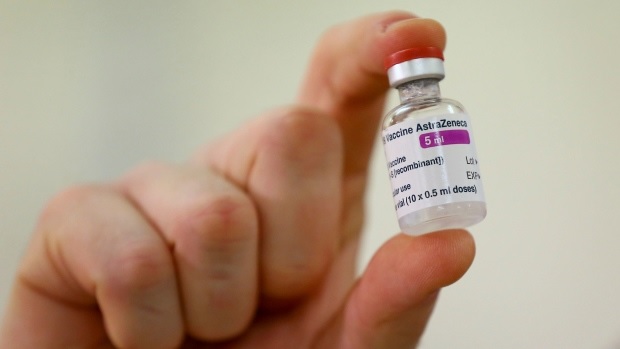
In this Jan. 2, 2021 file photo a vial of the COVID-19 vaccine developed by Oxford University and U.K.-based drugmaker AstraZeneca is checked after it arrived at the Princess Royal Hospital in Haywards Heath, England. (AP Photo/Gareth Fuller/Pool/File)
The report comes a day after Prime Minister Justin Trudeau announced a plan to produce millions of COVD-19 shots of the Novavax vaccine later this year at a plant in Montreal–a big step in securing a domestic supply in a volatile world market.
As well, the European Commission said yesterday its export controls will see ‘very limited’ use and that it has already authorized a vaccine delivery to Canada
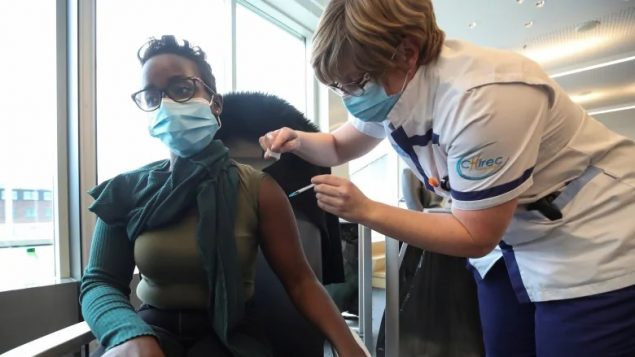
A medical staff member receives a dose of the Pfizer-BioNTech coronavirus disease vaccine earlier this month at the CHIREC Delta Hospital in Brussels, Belgium. A spokesperson for the European Union says that it has recently announced COVID-19 vaccine export controls will be limited in use and that the EU has already approved a vaccine shipment to Canada. (Reuters/Yves HeraBut the rollout–to understate–has not gone well.
In a report released last Wednesday, the U.K.-based Economist Intelligence Unit predicted that the majority of Canadians may have to wait six months longer than Americans and Europeans for their COVID-19 vaccine
Whether or not Canada’s luck is changing in the volatile world for vaccine procurement remains to be seen.
If it is, it can’t come soon enough for most Canadians, including–very likely–Trudeau and Anand.
As the pandemic unfolded, Ottawa struck deals with seven pharmaceutical firms for options to purchase up to 414 million doses–more than enough to vaccinate the 33 million adults and teenagers who live in Canada.
But the rollout has not gone well as both Pfizer-BioNTech and Moderna cut back on their deliveries.
In an assessement last Wednesday, the U.K.-based Economist Intelligence Unit predicted that the majority of Canadians may have to wait six months longer than Americans and Europeans for their COVID-19 vaccine.
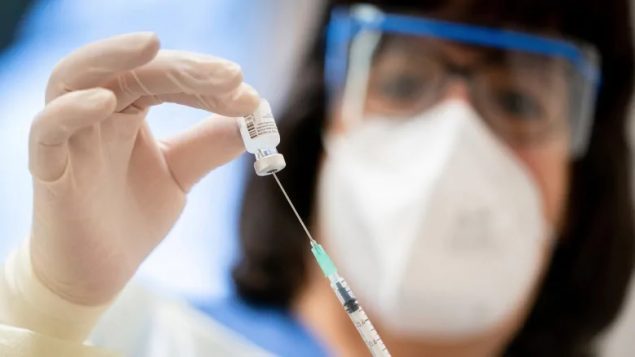
A nurse prepares a syringe with the BioNTech/Pfizer vaccine at the Bethel Hospital in Germany, on Jan. 13. (AP Photo/Kay Nietfeld)
According to data from the University of Oxford-based Our World in Data, Canada ranks 19th globally in the number of shots administered per capita–at 2.61 per 100 people in the total population.
Still, efforts to do better continue.
Last Friday, Novavax submitted an application to Health Canada for a rolling review of its vaccine–a process that allows for faster-than-usual approval once final results from clinical trials are complete.
Earlier last week, Providence Therapeutics, a Calgary-based company, announced that it had begun human trials for the first fully made-in-Canada COVID-19 vaccine.
If the results of the human trials are positive, Providence Therapeutics expects to have the vaccine ready at the end of 2021, or early 2022.
As of Tuesday night, 786,417 COVID-19 cases had been reported in Canada since the beginning of the pandemic.
Of those, 20,213 were fatal.
With files from CBC News, The Canadian Press (Mia Rabson)






For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.