Prime Minister Justin Trudeau says that everyone is going to have to wait patiently until the health situation allows Canada to loosen border restrictions internationally.
In a news conference in Montreal on Monday, Trudeau said there are ongoing discussions with the United States about the border restrictions.
He added that the first priority will always be ensuring the safety and security of Canadian citizens while also ensuring the flow of goods across the border.
“That’ll be eventually, but not for today,” Trudeau said.
The border between Canada and the U.S. has been closed to non-essential travel since March 2020.
Trudeau assures Canadians that AstraZeneca vaccine is safe
Trudeau also spoke about the concerns surrounding the AstraZeneca COVID-19 vaccine after its use has now been suspended in a number of European countries due to concerns about blood clots.
Germany, France, the Netherlands, Iceland, and Norway are just some of the countries that suspended use of the AstraZeneca vaccine.
“Health Canada and our experts and scientists have spent an awful lot of time making sure that every vaccine approved in Canada is both safe and effective,” Trudeau said.
“Therefore the best vaccine for you to take is the very first one that is offered to you. That is how we get through this as quickly as possible and as safely as possible.”
Trudeau said that health officials are following what’s happening with the specific batch of AstraZeneca vaccines used in Europe.
He also assured Canadians that none of the AstraZeneca doses Canada has received have come from that same batch.
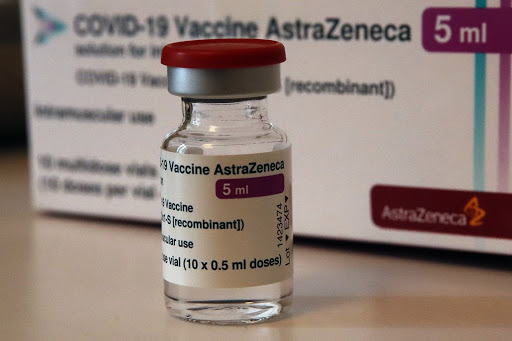
A vial of AstraZeneca vaccine is pictured in a pharmacy in Boulogne Billancourt, outside Paris, today. Germany, France and Italy on Monday became the latest countries to suspend use of AstraZeneca’s COVID-19 vaccine over reports of dangerous blood clots in some recipients. (AP Photo/Christophe Ena)
At the same news conference, Quebec Premier François Legault also said that there are no risks with the AstraZeneca vaccine.
In a press release on Sunday, AstraZeneca offered reassurance on the safety of its COVID-19 vaccine based on scientific evidence.
“A careful review of all available safety data of more than 17 million people vaccinated in the European Union and [the United Kingdom] with COVID-19 vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis (DVT) or thrombocytopenia, in any defined age group, gender, batch or in any particular country,” AstraZeneca said in a statement.
With files from CBC News (John Paul Tasker)







For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.