The western Canadian prairie province of Alberta is reported to have stopped administering first doses of the AstraZeneca vaccine against COVID-1. except in certain special cases. This includes Covishield which is the name of the same product produced in India.
The report was carried today in the influential Globe and Mail newspaper.
Other provinces have made no decision regarding the vaccine which is known in very rare cases to cause blood clots. There have been a few cases reported of blood clots from the vaccine in Canada and three people have died here from clots believed to be associated with the vaccine; one in New Brunswick, one in Quebec, and one in Alberta.
Alberta has now stopped administering first doses of Oxford-AstraZeneca’s COVID-19 vaccine, except in limited cases, becoming the only government in Canada to make such a move as other provinces weigh their options with remaining doses of the vaccine.
Health officials at all levels of government have repeatedly said the protective benefits of the vaccine far outweigh the potential health risks and continue to insist people should take whatever COVID-19 vaccine is available to them.
Last week the National Advisory Committee on Immunization (NACI) stirred up firestorm of controversy when it appeared to counter the government policy by suggesting the PfizerBioNtech or Moderna vaccines may be “preferred” by people in some situations.
Both the AstraZeneca and Johnson & Johnson vaccines use viral vector technology, while the PfizerBioNtech or Moderna vaccines use a new mRNA technology which has not been linked to blood clotting.
The Globe and Mail report says the pause in administering the Astra Zeneca vaccine is because no future shipments to the province are expected and that the supply of other vaccines is increasing and they are now becoming more widely available. It also however mentions concern about the rare but serious side effects.
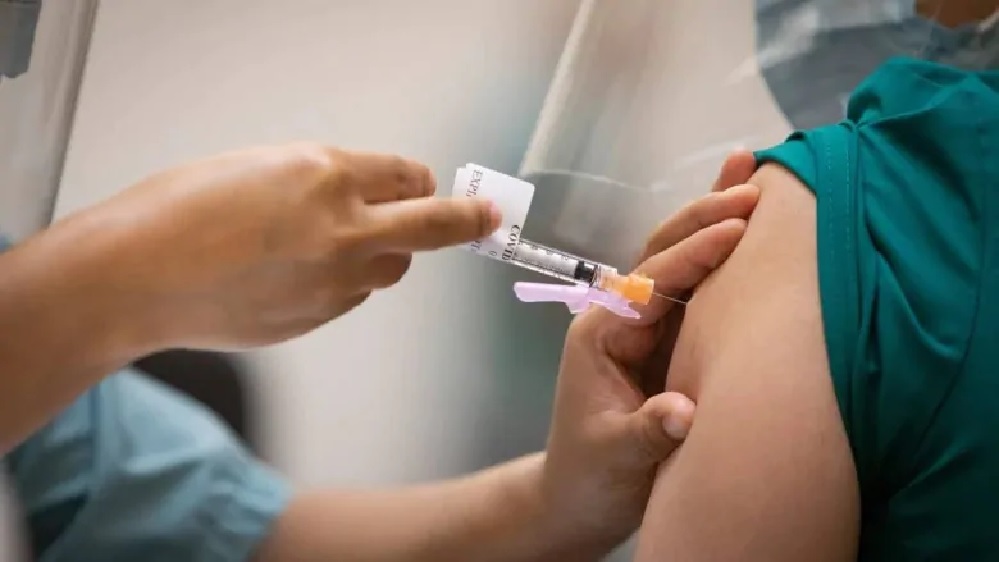
At least three countries have stopped administering the AstraZeneca vaccine. over safety concerns ( Evan Mitsui-CBC)
No other provinces in Canada have yet changed their policy, but medical officials elsewhere are reported to be reviewing the situation as they weigh the risks of the virus versus the risks of the Astra Zeneca/Covishield viral vector technology.
Quoted by the Globe and Mail, Kristin Klein, an Alberta medical officer of health and a co-lead of the province’s vaccine task force said, “Because we have so much mRNA vaccine, those are the vaccines that we are recommending for people to book their first dose with.”
She also said those who can’t wait for the mRNA products and who do want the AstraZeneca product can still get it.
All vaccines except the Johnson & Johnson product require two doses.
Canada is expecting over 600,000 more doses of the Astra Zeneca vaccine in coming weeks. Responding to Globe and Mail requests about future orders of that product, the federal health ministers office responded only that, “all vaccines authorized by Health Canada for use in Canada are safe and effective”.
Medical researchers in Britain, where the AstraZeneca product has been widely distributed, say the incidence of blood clots is about one in 100,000. This is the same figure cited by officials in Canada where about 2 million doses of the AZ vaccine have been given.
In Alberta some 255,000 people have received the first dose of the AstraZeneca vaccine and about 2,200 the second dose.
In March Norway decided to stop using the AZ vaccine and in a mid-April statement from the Norwegian Institute of Public Health said, “We now know significantly more about the association between the AstraZeneca vaccine and the rare but severe incidents with low platelet counts, blood clots and haemorrhages, (…) Based on this knowledge, we come with a recommendation to remove the AstraZeneca vaccine from the Coronavirus Immunisation Programme in Norway”.
Iceland and Denmark have also stopped using the AstraZeneca product and Britain, which has largely vaccinated with the vaccine is now suggesting against it for anyone under age 40.
Germany however is now saying any adult can get the AZ shot, and the J&J vaccine if given the go-ahead by their doctors.
According to the Globe and Mail, several Canadian doctors and officials are now expressing some degree of reservation about continuing administration of first doses of the AstraZeneca/Covishield vaccines.
Alberta Health spokesperson Tom McMillan said no future shipments of the Astra Zeneca are expected and that the province is receiving large shipments of the PfizerBioNtech and Moderna vaccines. He said the province will be holding the remaining 8,400 AstraZeneca doses on hand for administering a second shot to those who have already had one dose of that vaccine.
Researchers are still studying effects of mixing different vaccines for a first and second dose
additional information-sources
- Globe and Mail: May 11/21: Alberta stops giving first doses of Oxford AstraZeneca Covid-19 vaccine (PAYWALL)
- CTV: May 11/21: Alberta to reserve remaining Astra Zeneca stock for second doses due to supply concerns
- Global: M Gilligan: may 11/21: Alberta stops giving first doses of AstraZeneca Covid 19 vaccine
- Reuters: Mar 11/21: Denmark, Norway, and Iceland suspend AstraZeneca COVID shots after blood clot reports
- The Economic Times: May 10/21: Germany Ok’s J&J, Astra Zeneca jabs for all adults






For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.