Struggling to cope with the third wave of the COVID-19 pandemic, Canadian provinces of Ontario, Alberta and Manitoba will start offering the AstraZeneca-Oxford COVID-19 vaccine and its Indian-produced version, Covishield, to people age 40 and over.
In Quebec, where AstraZeneca is available to those between the ages of 55 and 79, Health Minister Christian Dube said provincial public health authorities were also considering whether to expand access.
The move to lower the age limit for the AstraZeneca vaccine from 55 to 40 comes despite the country’s National Advisory Committee on Immunization (NACI) recommendation to not give the vaccine to those under 55.
On Sunday, however, the federal government said the provinces and territories were free to expand eligibility for the AstraZeneca-Oxford vaccine to any adult over the age of 18 as some pharmacists warned they had doses sitting idle because of the age restrictions.
“Provinces and territories are free to use AstraZeneca in any population over 18 per Health Canada’s license for use in Canada,” federal Health Minister Patty Hajdu told reporters on Sunday.
The NACI’s recommendations were evolving based on the current evidence, she said.
“In fact, they [NACI] are reviewing AstraZeneca advice now and we’ll have an update in the near future,” Hajdu said.
The advisory committee first recommended the vaccine to not be given to those below 55 in late March, after reports of very rare but serious blood clotting side effects surfaced in several countries.
Dr. Shelley Deeks, the vice-chair of NACI, said that with “substantial uncertainty” around cases of vaccine-induced thrombocytopenia (VIPIT) in people with low platelets, the committee is recommending the suspension of shots in all people under 55 as a “precautionary measure.”
Based on early research out of Europe, VIPIT seems to be extremely rare. The odds of getting a blood clot are estimated to be between one in 100,000 and one in 250,000.
Alberta’s Chief Medical Officer of Health Dr. Deena Hinshaw said in comparison, people 55 and older who are diagnosed with COVID-19 have a one-in-200 chance of dying from that infection. They are also at least 1,500 times more likely to be hospitalized from COVID-19, Hinshaw said Sunday.
Two cases of rare blood clots caused by the AstraZeneca vaccine have been confirmed in Canada so far. Canada has received 2,315,900 doses of the vaccine from various sources, according to Health Canada.
Ontario desperate for more vaccine doses
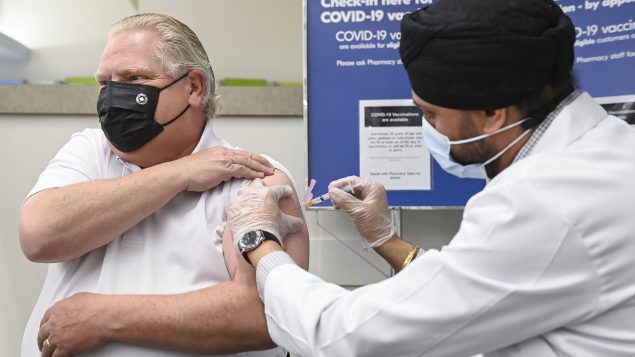
Ontario Premier Doug Ford, left, receives the Astrazeneca-Oxford COVID-19 vaccine from pharmacist Anmol Soor at Shoppers Drug Mart during the COVID-19 pandemic in Toronto on Friday, April 9, 2021. (Nathan Denette/THE CANADIAN PRESS)
But the office of Ontario Premier Doug Ford announced Monday that they’ve been warned by federal authorities of possible delays affecting two shipments of the AstraZeneca vaccine to the province later this month and next.
“As we look to expand our rollout of AstraZeneca to younger age groups and into more pharmacies, any delays to vaccine shipments would be devastating for Ontario right now as we battle the third wave of this pandemic,” the statement said.
Ford is “redoubling his efforts to secure more vaccines” by directly reaching out to Canada’s international allies for any available supply, it added.
“As part of that outreach, he has already spoken with Canada’s ambassador to Denmark and the Consulate General of the United States, both of whom are advancing our request to purchase any additional supply of AstraZeneca to their respective administrations,” the statement said.
“Our officials have already reached out to Norway’s ambassador, and the Premier will be speaking to the EU ambassador to Canada as well as the High Commissioner of India later today to ask for any extra AstraZeneca vaccines.”
Last week health authorities in Denmark announced that they will stop using AstraZeneca’s COVID-19 vaccine altogether over a potential link to the rare but serious form of blood clot.
Norway halted vaccinations with the AstraZeneca vaccine on March 11 after reports of possible serious side effects surfaced in several countries. Four people have died in Norway from a rare combination of blood clots, low platelets and bleeding following their vaccination shots.
With files from CBC News and The Canadian Press






For reasons beyond our control, and for an undetermined period of time, our comment section is now closed. However, our social networks remain open to your contributions.